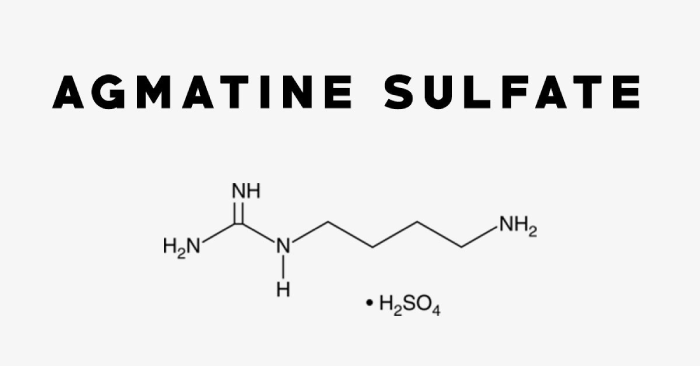
Foundation Medicine’s FoundationOne®Liquid CDx
Foundation Medicine, Inc. has announced that its FoundationOne®Liquid CDx has received approval from the U.S. Food and Drug Administration (FDA) as a companion diagnostic for TEPMETKO® (tepotinib). This approval, which includes use in both the United States and Canada, enables the FoundationOne Liquid CDx test to identify patients with metastatic non-small cell lung cancer (NSCLC) who may benefit from treatment with TEPMETKO. TEPMETKO, developed by EMD Serono, the healthcare arm of Merck KGaA, Darmstadt, Germany, received accelerated approval from the FDA in February 2021, followed by traditional approval in February 2024. This marks an important milestone for precision medicine, as FoundationOne Liquid CDx becomes the first FDA-approved companion diagnostic specifically for TEPMETKO.
NSCLC, the most common form of lung cancer, accounts for approximately 85% of all lung cancer diagnoses. Of these, 3-4% are linked to MET exon 14 skipping alterations (METex14), a genetic mutation associated with aggressive cancer progression and poor prognosis. The ability to identify these alterations early is crucial for effective treatment, and the new approval of FoundationOne Liquid CDx will help in the identification of patients who are candidates for targeted therapy with TEPMETKO.
“Access to a high-quality liquid biopsy, such as FoundationOne Liquid CDx, is pivotal for unlocking the power of precision medicine, allowing for a more tailored approach to treatment,†said Dr. Mia Levy, Chief Medical Officer at Foundation Medicine. “We are proud that our liquid biopsy test is the first companion diagnostic approved for TEPMETKO in the U.S., making it easier to identify patients with METex14 alterations who may benefit from targeted treatment.”
FoundationOne Liquid CDx, which uses a blood sample to perform genomic profiling, analyzes more than 300 cancer-related genes to provide insights into the genomic landscape of the tumor. This non-invasive method offers a significant advantage over traditional tissue biopsies, which can be invasive and difficult for some patients to undergo. With this approval, Foundation Medicine now boasts more than 19 FDA-approved companion diagnostic indications for NSCLC, more than any other company in this space.
The approval also highlights the growing importance of biomarker testing in the treatment of cancer. Laurie Ambrose, President and CEO of GO2 for Lung Cancer, emphasized the critical role that targeted therapies and biomarker testing play in improving outcomes for lung cancer patients: “Biomarker testing is key to ensuring that patients receive the right treatments. We’re thrilled to see more patients with advanced NSCLC gaining access to targeted therapies thanks to the use of non-invasive liquid biopsies.â€
Foundation Medicine’s market leadership is further affirmed by its status as the global leader in approved companion diagnostic indications, accounting for 50% of all FDA-approved next-generation sequencing (NGS) companion diagnostics in both the United States and Japan.
Commentary by YourDailyFit columnist Alice Winters

The recent FDA approval of FoundationOne®Liquid CDx as a companion diagnostic for TEPMETKO® marks a significant step forward in the realm of personalized cancer treatment, particularly for patients with metastatic non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping alterations (METex14). This development underscores the growing importance of liquid biopsy tests in the oncology space, where precision medicine continues to gain traction.
One of the most notable aspects of this approval is the pivotal role that liquid biopsy technology plays in advancing cancer care. Traditional biopsies, while effective, are invasive, often requiring surgical intervention or the collection of tissue from hard-to-reach areas. Liquid biopsy, on the other hand, uses a simple blood sample to perform an in-depth analysis of cancer-related genes, offering a non-invasive and far less painful alternative. This opens up new possibilities for patients, especially those who may not be candidates for traditional biopsies due to the location or extent of their tumors. Moreover, the ability to gather genomic insights from a blood sample significantly reduces the risk of complications and discomfort, which is crucial for patients already battling advanced stages of cancer.
In addition to the practical benefits, the approval of FoundationOne Liquid CDx highlights the power of targeted therapies in the treatment of cancer. By identifying specific genetic alterations like METex14, this diagnostic test can pinpoint patients who are most likely to benefit from TEPMETKO, a targeted drug that works to inhibit the MET receptor, a key driver of cancer progression in this subset of patients. This is a classic example of how precision medicine is shifting the paradigm in cancer treatment, moving away from a one-size-fits-all approach to more individualized, tailored therapies.
From a market perspective, Foundation Medicine’s approval is yet another testament to its leadership in the field of genomic profiling. The company’s ability to secure over 19 FDA-approved companion diagnostic indications for NSCLC, the highest in the industry, speaks volumes about its expertise and commitment to advancing cancer treatment. Additionally, FoundationOne Liquid CDx’s ability to analyze over 300 cancer-related genes offers a comprehensive tool for identifying not just METex14 alterations, but a range of genetic mutations that could guide treatment decisions. This broad scope enhances the test’s value for clinicians, enabling them to make informed, data-driven decisions about the best course of treatment for their patients.
However, it’s worth noting that while the approval is a major advancement, the real-world impact will ultimately depend on how accessible and widely adopted these liquid biopsy tests become. Despite their advantages, liquid biopsies can be expensive, and there may be challenges surrounding insurance coverage and reimbursement. The affordability and accessibility of these tests will play a crucial role in ensuring that the benefits of precision medicine reach a broader patient population. Furthermore, as liquid biopsy technology continues to evolve, ongoing research will be necessary to refine the sensitivity and accuracy of these tests, ensuring that they can detect a broad range of genetic mutations with minimal risk of false positives or negatives.
In summary, FoundationOne Liquid CDx’s FDA approval for use with TEPMETKO represents a meaningful leap forward for precision oncology. It is a reflection of the growing shift toward personalized treatment in cancer care, one that promises to deliver more effective, targeted therapies and ultimately improve outcomes for patients with NSCLC. As this technology matures and becomes more widely accessible, it has the potential to transform cancer treatment and reshape the landscape of oncology for years to come.