A New Standard in Targeted Therapy for Metastatic Breast Cancer
Daiichi Sankyo and AstraZeneca’s ENHERTU® (fam-trastuzumab deruxtecan-nxki) has secured approval from the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer. Eligibility requires confirmation through an FDA-approved diagnostic test, and the treatment is indicated for patients who have experienced progression following one or more endocrine therapies in the metastatic setting.
A Precision-Engineered Antibody Drug Conjugate
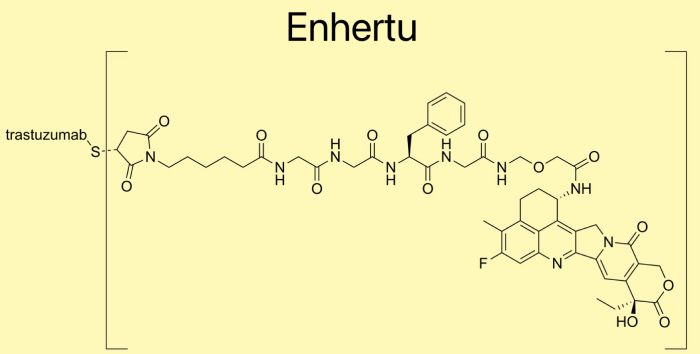
ENHERTU is a HER2-targeted DXd antibody-drug conjugate (ADC) developed by Daiichi Sankyo in collaboration with AstraZeneca. The approval follows the presentation of pivotal results from the DESTINY-Breast06 Phase 3 trial at the 2024 American Society of Clinical Oncology (#ASCO24) Annual Meeting and a publication in The New England Journal of Medicine. The FDA granted Priority Review and Breakthrough Therapy Designation for ENHERTU in this treatment setting, underscoring its significance.
Clinical Trial Results: Superior Efficacy Over Chemotherapy
The DESTINY-Breast06 trial assessed the efficacy of ENHERTU in chemotherapy-naïve HR-positive, HER2-low, or HER2-ultralow metastatic breast cancer patients (n=866). The study revealed a 36% reduction in disease progression or mortality risk compared to standard chemotherapy (hazard ratio [HR] 0.64; 95% confidence interval [CI]: 0.54-0.76; p<0.0001).
Progression-Free Survival (PFS): ENHERTU-treated patients experienced a median PFS of 13.2 months (95% CI: 12.0-15.2), compared to 8.1 months (95% CI: 7.0-9.0) in the chemotherapy group.
Objective Response Rate (ORR): The ORR was 62.6% (95% CI: 57.6-67.4) in the ENHERTU group versus 34.4% (95% CI: 29.7-39.4) in the chemotherapy arm, with 10 complete responses (2.5%) and 236 partial responses (60.1%).
Duration of Response (DOR): ENHERTU-treated patients achieved a median DOR of 14.3 months (95% CI: 12.5-15.9), compared to 8.6 months (95% CI: 6.9-11.5) with chemotherapy.
Performance Across HER2 Expression Levels
Among HER2-low patients (n=713), median PFS was 13.2 months in the ENHERTU group versus 8.1 months in the chemotherapy group (HR 0.62; 95% CI: 0.52-0.75; p<0.0001). The ORR was 62.0%, and median DOR reached 14.1 months.
In an exploratory HER2-ultralow subgroup analysis (n=153), median PFS was 15.1 months with ENHERTU versus 8.3 months for chemotherapy (HR 0.76; 95% CI: 0.49-1.17). The ORR in this group was 65.7%, with a median DOR of 14.3 months.
Expert Perspectives on ENHERTU’s Impact
Dr. Aditya Bardia, MD, MPH, Program Director of Breast Oncology at UCLA Health, emphasized the potential paradigm shift:
“With a median progression-free survival exceeding one year and a response rate above 60%, trastuzumab deruxtecan presents a promising new standard of care for HR-positive, HER2-low, or HER2-ultralow metastatic breast cancer following endocrine therapy.”
Krissa Smith, Vice President of Education at Susan G. Komen, highlighted the importance of precise biomarker testing in guiding treatment decisions.
Safety Considerations and FDA Boxed Warnings
ENHERTU carries Boxed Warnings for:
- Interstitial lung disease (ILD)/pneumonitis
- Embryo-fetal toxicity
Among 434 patients assessed in the DESTINY-Breast06 trial, common (≥20%) adverse events included:
- Decreased white blood cell and neutrophil counts
- Nausea, fatigue, anemia, and platelet reduction
- Liver enzyme elevations and electrolyte imbalances
- Gastrointestinal symptoms (diarrhea, vomiting, constipation)
- Musculoskeletal pain and alopecia
Serious adverse reactions occurred in 20% of patients, with 2.8% experiencing fatal complications, predominantly ILD/pneumonitis, COVID-19, febrile neutropenia, and hypokalemia.
Industry Leaders Weigh In
Ken Keller, Global Head of Oncology Business at Daiichi Sankyo, described the approval as a landmark achievement:
“ENHERTU is redefining the classification and treatment landscape for HR-positive metastatic breast cancer, signaling another shift in treatment paradigms.”
Dave Fredrickson, Executive Vice President of AstraZeneca’s Oncology Hematology Business Unit, stressed the necessity of biomarker testing:
“This approval highlights the need for standardized HER2 testing in metastatic breast cancer to ensure patients receive optimal treatment strategies.”
Commentary by SuppBase Columnist Alice Winters
ENHERTU’s approval represents a significant milestone in oncology, yet its broader implications warrant critical examination. The treatment’s efficacy, particularly its superior ORR and prolonged PFS over chemotherapy, underscores its potential as a frontline option following endocrine therapy failure. However, several key considerations remain:
1. Efficacy in Real-World Settings
While DESTINY-Breast06 showcases impressive clinical results, real-world effectiveness may vary due to patient heterogeneity and comorbidities. Further post-market surveillance is crucial to validate these outcomes across broader demographics.
2. Adverse Event Burden
The toxicity profile of ENHERTU, particularly its ILD risk, poses a significant safety concern. Clinicians must carefully balance the benefits of prolonged PFS against the potential for severe, even fatal, adverse reactions. The high rate of neutropenia and gastrointestinal effects further complicates patient management.
3. Economic and Accessibility Barriers
The pricing of ENHERTU remains undisclosed but is expected to be substantially higher than standard chemotherapy. For patients without robust insurance coverage, cost considerations could severely limit access. Moreover, HER2 biomarker testing requirements add another layer of expense and logistical complexity.
4. Market Implications and Competitive Landscape
With multiple HER2-targeted ADCs in development, including those from Seagen and Gilead, competition in this segment will intensify. The approval of ENHERTU in earlier treatment settings further pressures existing therapies to demonstrate superior outcomes.
Conclusion
ENHERTU undeniably advances the treatment landscape for HR-positive, HER2-low breast cancer. However, safety concerns, real-world applicability, cost, and competition necessitate careful scrutiny. Clinicians and patients must weigh the benefits against potential risks while awaiting further long-term data.