Kidney Drug: Do Cinacalcet Tablets Contain Carcinogen?
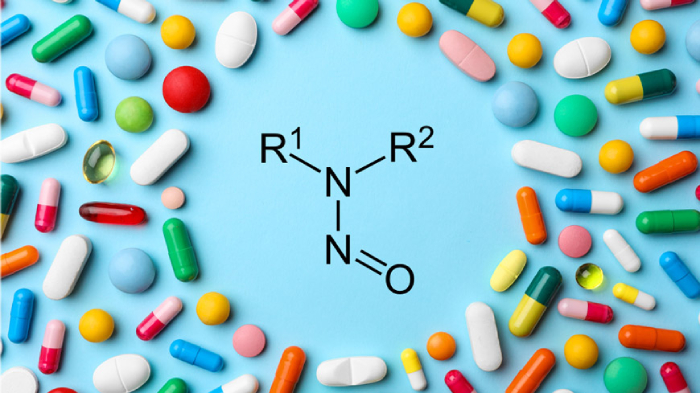
A major recall has been issued for over 300,000 bottles of a widely used kidney drug in the United States due to contamination concerns. Cinacalcet tablets, produced by Dr. Reddy’s Laboratories, have been found to contain potentially harmful levels of nitrosamine, a compound that is considered a possible carcinogen. The U.S. Food and Drug Administration (FDA) has announced that 331,590 bottles of these tablets, distributed under the Dr. Reddy’s brand, are being pulled from the market.
The FDA clarified that while low levels of nitrosamine are naturally found in some foods and beverages, prolonged exposure to higher levels of this chemical—especially in pharmaceuticals—can pose serious health risks, including cancer. As a result, the agency has urged patients to stop using the affected drugs immediately and to consult their healthcare providers for alternative treatments.
The recall is classified as a “Class II” risk, meaning that it may cause temporary or medically reversible health issues, but it is not expected to result in life-threatening conditions. The contaminated cinacalcet tablets, which come in 30mg, 60mg, and 90mg doses, were sold in bottles containing 30 tablets. These were manufactured by Dr. Reddy’s Laboratories in India, but marketed under the company’s U.S. label.
The recall affects a significant portion of the population, as cinacalcet is prescribed to approximately 23% of patients with chronic kidney disease in the United States. It is primarily used to manage hyperparathyroidism in patients undergoing dialysis, a condition in which the parathyroid glands secrete excessive parathyroid hormone (PTH), which in turn causes dangerous imbalances in calcium and phosphorus levels. By reducing PTH levels, cinacalcet helps to manage these imbalances, which can otherwise lead to complications like kidney stones and bone loss.
The recall follows concerns over the manufacturing process, as nitrosamines can be introduced at various stages, including during production, storage, or even distribution. Dr. Reddy’s Laboratories, which has been in operation since 1984 and has over 20 manufacturing facilities worldwide, has now been tasked with addressing the issue. The company first introduced cinacalcet in the U.S. market in 2000, where it competes with Sensipar, a similar product by Amgen, which generates hundreds of millions in annual sales.
This recall underscores the growing risks associated with pharmaceutical manufacturing, especially as more and more companies shift production to facilities in different parts of the world. It also highlights the importance of vigilant regulatory oversight in the drug industry, particularly when dealing with products used by large, vulnerable populations, such as those with chronic kidney disease.
Commentary by YourDailyFit columnist Alice Winters

The recall of Dr. Reddy’s cinacalcet tablets is a stark reminder of the critical need for stringent quality control in pharmaceutical manufacturing. Nitrosamines, while present in many foods, are notoriously hard to eliminate once introduced into the drug supply chain. Their potential carcinogenic properties elevate the stakes for manufacturers and regulators alike, particularly when dealing with drugs used by populations with specific health vulnerabilities, such as kidney disease patients.
Manufacturing Oversight and Supply Chain Concerns
The issue of nitrosamine contamination is not unique to Dr. Reddy’s but is part of a broader trend in the pharmaceutical industry. Over the past few years, several drug recalls have been issued for similar concerns, particularly with products manufactured overseas. The presence of nitrosamines can often be traced back to specific stages of the manufacturing process, such as the choice of raw materials, storage conditions, or even the chemicals used during production. The global nature of pharmaceutical manufacturing means that risks are often shared across borders, and ensuring that these risks are managed at every point in the supply chain is more important than ever.
The Vulnerability of Kidney Disease Patients
The recall also highlights the challenges faced by kidney disease patients, who rely on medications like cinacalcet to manage complex conditions like hyperparathyroidism. For the approximately 23% of patients with chronic kidney disease who take cinacalcet in the U.S., the recall represents an additional layer of uncertainty, as they may need to quickly transition to alternative treatments. This presents an immediate concern for healthcare providers and patients alike, as they will now have to assess the safety of other therapeutic options available.
Health Risks and Regulatory Oversight
While the recall has been classified as “Class II,” indicating that the potential health effects are reversible, the risk of prolonged exposure to nitrosamines in medications cannot be underestimated. The FDA’s recommendation for patients to stop taking the affected drugs and seek professional advice is prudent, yet the presence of carcinogens in routine medications is an issue that transcends this specific recall. It brings to light questions regarding the consistency of oversight in ensuring that pharmaceutical companies adhere to the highest safety standards.
Brand Impact and Market Dynamics
Dr. Reddy’s Laboratories, which has built a reputation as a cost-effective alternative to brand-name drugs, may face significant challenges in restoring consumer confidence following this recall. While the company has a broad portfolio of drugs, including a global presence, this recall could tarnish its standing among both healthcare providers and patients. The competitive market for kidney disease treatments, including cinacalcet, is intense, with players like Amgen’s Sensipar commanding substantial market share. In the wake of this recall, consumers may be more cautious about switching to or continuing treatment with generic alternatives, which could ultimately impact Dr. Reddy’s long-term growth prospects in this space.
In conclusion, while the recall of cinacalcet tablets is an isolated incident, it underscores ongoing challenges within pharmaceutical manufacturing, particularly regarding the presence of contaminants and the vulnerability of patients dependent on these critical drugs. As patients and healthcare professionals navigate the fallout from this recall, the industry must look inward at the adequacy of its safety protocols and work toward greater transparency and accountability.



